In our previous blog, we talked about the optical properties that make rocks and crystals a wonderful sight to behold. We learned that colour, lustre, transparency, and behaviours of light are all vital factors in deciding a gem's real worth and value.
Now let's draw our attention to another basic gemology topic: their structure. This refers to a crystal's internal and external shape. Structure matters because it defines our experience with handling and using a crystal for a wide range of purposes.
Let's dig in!
What Are Crystalline and Noncrystalline Solids?

Most minerals are crystalline. Meaning, they are solid crystals with a structured arrangement of atoms. When a mineral is a crystal, it has a geometric shape and has flat surfaces. Crystalline solids are classified into isotropic and anisotropic.
Isotropic - These minerals have symmetrical atomic arrangements. They're made up of atoms that are arranged in a cubic pattern. Examples of isotropic minerals are fluorite, diamond, gold, pyrite, sodalite, and spinel.
Anisotropic - The other side of the crystalline spectrum are anisotropic minerals whose properties vary in different directions. Their crystals may not be as symmetrical as the cubic crystals of fluorite, but their crystal structure is symmetrical nonetheless. Examples are barite, topaz, and olivine with well-formed long and tabular crystals.
There are also substances with no definite crystal shape due to an absence of a fixed atomic structure. These mineral-like substances are called noncrystalline or amorphous solids. Some common amorphous minerals are obsidian, opal, and jet.
Most amorphous solids are isotropic, so they show the same physical and optical properties in all directions.
How Do Crystals Form?

Have you ever wondered how your gorgeous gems occur in nature? Crystal formation happens when constituent atoms are brought together and arrange themselves in a repetitive pattern. A crystal grows from a tiny molecule and on to its final visible form.
The growth process is influenced by a variety of elements and environmental settings. But for growth to take place, these 3 mechanisms should be present:
- Crystals developing with the cooling of magma
- Crystals precipitating from water
- Crystals growing as a result of chemical reactions
Below are some types of mineral crystals based on the environments where they grow:
Igneous
Almost all minerals that form in igneous rocks are silicates. This is because magmas that create igneous rocks mostly consist of oxygen and silicon.
Other elements present in this environment are aluminum, calcium, magnesium, iron, sodium, and potassium. Given the right conditions, crystals formed in igneous rocks may grow prominent crystal faces.
When minerals crystallize simultaneously and they have enough room to grow as individual crystals, they can form a mosaic colour pattern. This is the case with feldspar and olivine.
Some magmas cool slowly so crystals grow to be large specimens. The largest crystals in the world have been extracted from pegmatites (coarse-grained igneous rocks). Example of this is the quartz crystal found in Brazil which weighed beyond 5 tons.
Some crystals can be so small that they are invisible to the naked eye. Igneous crystals can vary diversely due to differences in igneous processes.
Aqueous minerals
From the word aqueous, these minerals precipitate from hot water flowing underground, the evaporation of a lake or inland seas, or directly from seawater. This precipitation happens due to changes in chemical composition, temperature, pressure, and pH levels.
Aqueous crystals like calcite, gypsum, and halite are usually produced in inland lakes or seas. Examples of these locations include The Dead Sea near Jordan and Israel. Geodes may also form through aqueous solutions.
Hydrothermal minerals
These minerals (e.g. oxides and sulfides) are produced underground when hot groundwater deposits minerals and makes an ore deposit. Minerals can be found in veins, vugs, or throughout a rock.
Hydrothermal minerals often come in vivid colours because they contain transition metals. They have a metallic lustre and have symmetrical crystals. Examples are blue azurite, green malachite, sphalerite, and pyrite.
The 6 Crystal Systems
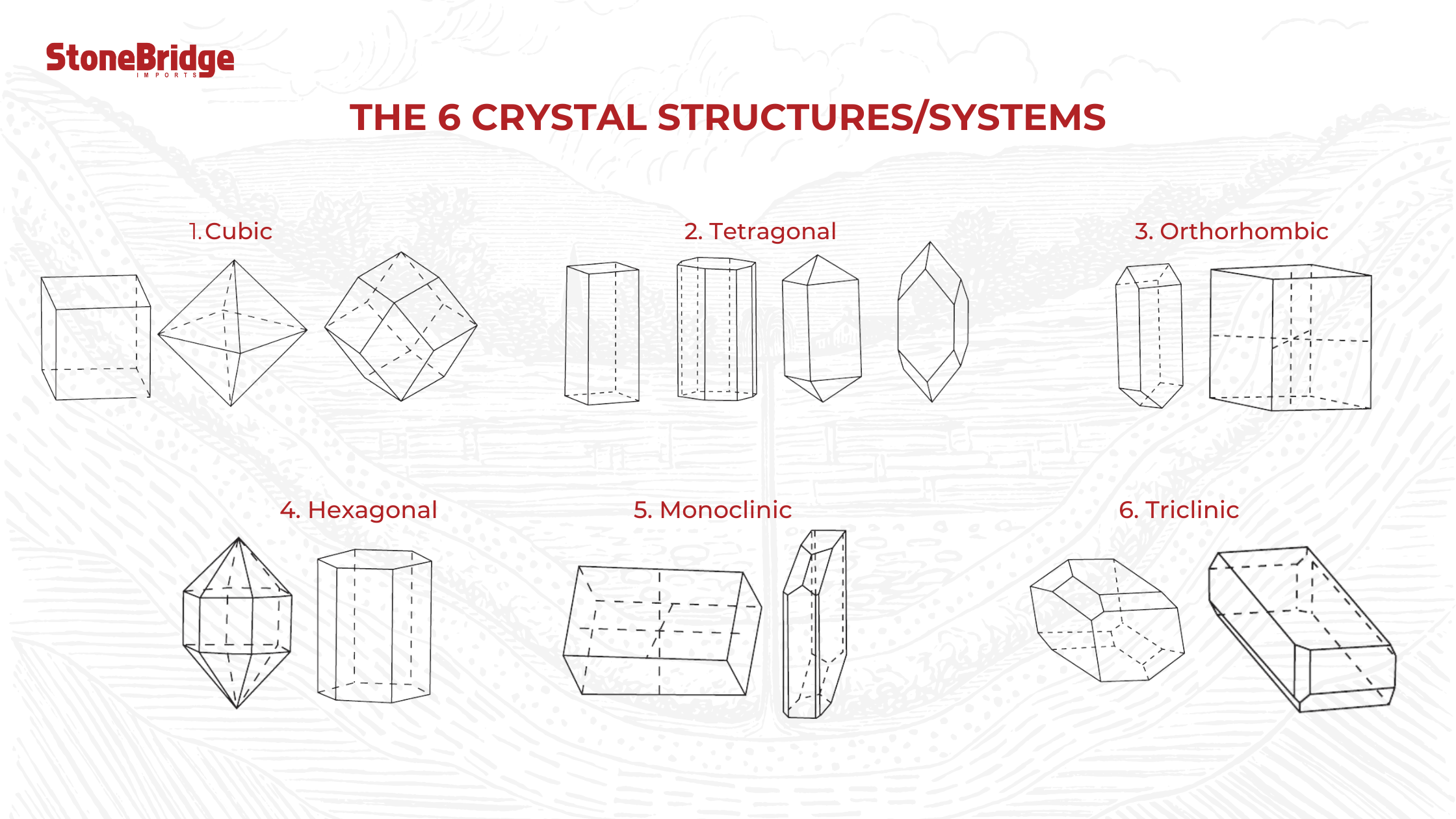
Crystalline minerals grow into one of the 6 main crystal systems. These systems determine the ordered arrangement of atoms, ions, or molecules in the crystal.
Each system has the following factors: the number of axes (the direction between the sides) it has, the lengths of the axes, and the angles at which the axes meet.
The crystal systems are:
1. Cubic - A shape consisting of 6 square faces at 90 ° to each other and 3 axes. Diamond, garnet, and spinel have a cubic crystal structure.
2. Tetragonal - A tetragonal crystal has 3 axes that meet at 90 °. Apophyllite, rutile, and zircon have a tetragonal crystal system.
3. Orthorhombic - This system has 3 axes which all meet at 90 °. The axes have varying lengths. Examples are alexandrite, chrysoberyl, peridot, topaz, and tanzanite.
4. Hexagonal - The hexagonal crystal system has 4 axes, 3 of these are equal in length and intersect at 120 °. Its vertical axis or the C axis is longer and meets the other shorter axes at 90 °. Apatite, aquamarine, beryl, emerald, and morganite have a hexagonal crystal.
5. Monoclinic - Two of its axes namely A and C meet at 90 °, except axis B. Each of its axes have varying lengths (imagine a matchbox that slants to 1 side). Azurite, lazulite, jadeite, malachite, and staurolite form in the monoclinic system.
6. Triclinic - in the triclinic system, all axes have different lengths. None of them intersect at 90 °. Imagine a matchbox which slants to 2 sides. Examples of these minerals are amazonite, kyanite, labradorite, and turquoise.
What Are Crystal Habits?
If a crystal system refers to the repetitive patterns of atoms in a crystal, a crystal habit defines the external shape of a mineral or group of minerals. A habit can be influenced by the crystal system and the growth environment of a mineral.
These crystal habits were named by mineralogists for ease of description:
- Acicular - Needle-like
- Druzy - Small crystals coating a surface
- Fibrous - Showing thread-like fibres
- Coxcomb - Closely-spaced, flaky crystals
- Tabular - Flattened or tablet-shaped
- Plumose - Feathery
- Sphenoid - Wedge-like
- Mammillary - Containing many smoothly rounded convex surfaces
- Euhedral - Crystals with well-formed faces
What Factors Affect Crystal Size and Shape?
The most essential factors that control a crystal's size and overall quality are time, temperature, the abundance of important elements, and the presence/absence of a flux. These factors interact together to form a large, perfectly shaped crystal.
1. Time - The duration of a crystal's growth determines its size. A longer time to grow means a crystal can grow larger and better ordered, since more atoms will transfer to the growing crystal and arrange themselves in a regular pattern.
This is what happens with intrusive igneous rocks. They have coarser grains than extrusive igneous rocks because they cool slowly underground.
2. Temperature - Thermodynamics 101 tells us that crystals that are produced at high temperatures tend to have simpler atomic structures compared to crystals that grow at low temperatures. This is another reason why large and well-formed crystals are found in high-temperature environments.
3. Elements - Although the time and temperature are right, a mineral cannot form into a large crystal if the needed elements are not present.
On average, a rock needs up to a dozen elements to form. Minerals consisting of those elements are typically larger than those made of rarer elements.
4. Presence/absence of a flux - The diffusion of atoms through solid is slow. Also, atoms may not migrate as fast to areas where crystal growth is occurring. But when a hydrothermal fluid is present, it can act as a flux that carries atoms to spots of crystallization.
Crystal Imperfections

Have you noticed how some raw crystals have odd shapes that you just can't explain?
Some minerals consist of 2 or more crystals, one slightly bigger than the other and each situated in a weird position. Others have an even more obscure and indiscernible shape. This is a real deal breaker for some collectors, but others love their specimens just the same despite the form.
Minerals don't always grow in ideal conditions, and this causes them to grow imperfect crystals. There are 2 common imperfections that result during or after crystal formation:
1. Zoning - Crystal zoning is caused by changes in temperature or pressure during crystallization. It can also happen due to changes in fluid or magma composition during the process of crystal growth.
Often, zoning forms as growth rings around the original crystal seed. Other times it is more complex that its occurrence can be difficult to explain.
Zoning is quite common in several minerals, but it can be invisible to the naked eye. Some minerals have it large and in different colours. Many zones have different compositions and therefore have different optical properties. Zoning often occurs in fluorite and tourmaline crystals.
2. Twinning - In an ideal setting, the atoms of a crystal are arranged in a repetitive order in all parts of the crystal. But this doesn't happen all the time.
Some crystals have different atomic orientations, and this affects the characteristic shape of a crystal. Thus, the formation of contact and penetration twins.
These are the usual causes of twinning:
During growth - As a crystal grows, magmatic changes such as pressure, temperature, and flow can happen, altering a crystal's orientation.
When they are added outside of an already existing crystal, new atoms are misplaced and all other new atoms are arranged in another orientation than the original crystal. This leads to the formation of a new crystal which is adjacent to the original one.
The resulting new crystal is called a contact twin if a common plane of atoms is oriented correctly for old and new crystals. It's a penetration twin if a common volume of atoms is oriented correctly for both old and new crystals.
Transformation - This happens when the atomic arrangement of a full grown crystal is altered due to changes in temperature and pressure. For example, a hexagonal quartz crystal is called beta-quartz at a temperature of 573 °C and above.
If the temperature dips, beta-quartz will turn into trigonal alpha-quartz. And twinning will happen during this transformation.
Deformation - When a fully-grown crystal is under stress or pressure, deformation twins occur. The pressure distorts the crystal lattice which causes crystal domains to be oriented differently. This twinning phenomenon is common in calcite.
Twinning occurs in different forms and shapes. It is visible in some minerals, but in others you can only detect it using a microscope. Other cases of twinning cannot be seen even with the aid of a more advanced apparatus.
Sources:
Crystallography. (2019, June 23). Geo.libretexts.org. Accessed at https://geo.libretexts.org/Bookshelves/Geology/Book%3A_Gemology/05%3A_Crystallography
Crystals and Crystallization. (n.d.). Opengeology.org. Accessed at https://opengeology.org/Mineralogy/4-crystals-and-crystallization/#444_Crystal_Defects
The Colors of Gemstones. (n.d.). Scifun.org. Accessed at http://www.scifun.org/chemweek/ColorOfGemstones2017.pdf
Boothe, R. (2019, June 17). How Geodes Form in the Middle West. Moment of Science. Accessed at ??https://indianapublicmedia.org/amomentofscience/geodes-form-middle-west.php
King, H. (n.d.). Crystal Habits and Forms of Minerals and Gems. Geology.com. Accessed at https://geology.com/minerals/crystal-habit/